NOBILIS® RISMAVAC+ CA 126
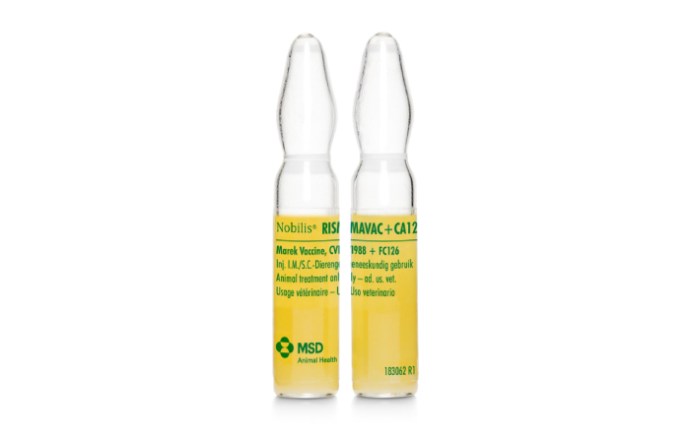
Nobilis® Rismavac + CA126 is a combined live, cell-associated Marek’s disease vaccine recommended for the immunization of healthy chicks against Marek’s disease.
Product Description
Advantages:
Marek’s disease vaccine combination of Rispen and low passage HVT with high titer provides protection against VVMD.

Indications:
Nobilis® Rismavac + CA 126 is a combination active cell associated vaccine against Marek’s disease which is recommended for immunization of healthy chicks against Marek’s disease. Indicated for the prevalence of very virulent Marek’s disease (vvMD) cases.

Content:
Each dose of Nobilis®Rismavac + CA 126 contained at least 3.0 log10 PFU HVT (Turkey Herpesvirus) live strain FC-126 (serotype 3) and at least 3.0 log10 PFU CHV (Chicken Herpesvirus) live strain CVl-988 (serotype 1) in the form of cell-associated, as a virus suspension containing fibroblasts of chicken embryos SPF.
Dosing and Administration:
One dose of 0.05 ml was given to 18 days old embryonated eggs or 0.2 ml via intramuscular injection in the thigh or subcutaneous injection in the neck in day one old chicks. Use only with the recommended diluent CA (cell associated) diluent.

Presentation:
Ampoule 1000 and 2000 doses.

Storage:
AMPOULES – Store in a filled liquid nitrogen container.
DILUENT – Store at room temperature (15°C – 25°C).
CONTAINER – Store liquid nitrogen container securely in upright position in a clean, dry and well-ventilated room separated from the hatching/chicken room in the hatchery.

Registration holder:
PT Intervet Indonesia
KEMENTAN RI No. I 1608205 VKM.2
For animal use only.
Not all presentations may be marketed.
